| RealCyto Live/Dead Cell Dual Staining Kit | Package |
|---|---|
| Green Stock solution for live cell (Green label, brown vials) | Two vials, 4mg/mL, 50μL each |
| Red Stock solution for dead cell (Red label, brown vials) | Two vials, 20mg/mL, 50μL each |
| Quick Facts |
| Storage conditions before opening:<-20 ℃, Protect from light. |
| Ex/Em (nm):Live cell (485/530)、Dead cell (530/645). |
| Recommended dye concentration for staining working solution: 2μg/mL Green label &10μg/mL Red label (Dilution ratio:2000X). |
| The mixing ratio of cell sample and working solutions:1/ 1. |
Handling of Reagents
- Allow the reagents to warm to room temperature (about 30 mins) and centrifuge briefly before opening. Before refreezing, seal all stock solutions tightly.
- Green label fluorescent dye is sensitive to moisture and light. Prepare aqueous working solution immediately prior to use and use it right away. Red label is stable. The working solution of Red label can stored at -20 °C about one year.
Protocol
Fluorescence microscopes:
-
Prepare the Cells:
1.1 Adherent cells may be cultured on sterile glass coverslips as either confluent or subconfluent monolayers (e.g., fibroblasts are typically grown on the coverslip for 2-3 days until acceptable cell densities are obtained). The cells may be cultured inside 35 mm disposable petri dishes or other suitable containers; non-adherent cells may also be used.
1.2 Wash the cells prior to the assay to remove or dilute serum esterase activity generally present in serum-supplemented growth media (serum esterases could cause some increase in extracellular fluorescence by hydrolyzing calcein AM). Wash adherent cells gently with 500-1,000 volumes of Dulbecco’s phosphate-buffered saline (D-PBS)。
1.3 Wash non-adherent cells in a test tube with 500-1,000 volumes D-PBS and sediment by centrifugation. Transfer an aliquot of the cell suspension to a coverslip. Allow cells to settle to the surface of the glass coverslip at 37°C in a covered 35 mm petri dish.
1.4 Treat the cells with cytotoxic agents as required at any time prior to Live/Dead dual staining.
-
Dilution Protocol:10 mL of working solution (This is an example; the optimal dye concentrations are likely to vary depending on the cell type.)
2.1 Allow the reagents to warm to room temperature (about 30 mins) and centrifuge briefly before opening.
2.2 Add 5 μL of the supplied Red Stock solution to 10 mL of sterilized D-PBS, vortexing to ensure mixing thoroughly.
2.3 Combine the reagents by transferring 5 μL of the supplied Green Stock solution to the above 10 mL Red Stock working solution. Vortex the resulting solution to ensure mixing thoroughly.
2.4 The above working solution results approximately 2 μg/mL Green Stock and 10μg/mL Red Stock, then, added directly to cells. These working solutions should therefore be used within 2 hours.
-
Perform the Viability Assay:
3.1 Add 100–150 μL of the combined assay working solution (procedure 2) to the surface of a 22 mm square coverslip (procedure 1), so that all cells are covered with solution. Incubations should be performed in a covered dish (e.g., 35 mm disposable petri dish) to prevent contamination or drying of the samples. Incubate the cells for 30–45 minutes at room temperature.
3.2 Following incubation, add about 10 μL of the fresh working solution or D-PBS to a clean microscope slide. Using fine-tipped forceps, carefully andquickly invert and mount the wet coverslip (procedure 3.1) on the microscope slide. To prevent evaporation, seal the coverslip to the glass slide e.g., with clear fingernail polish. Avoid damaging or shearing cells in the preparation of the slides.
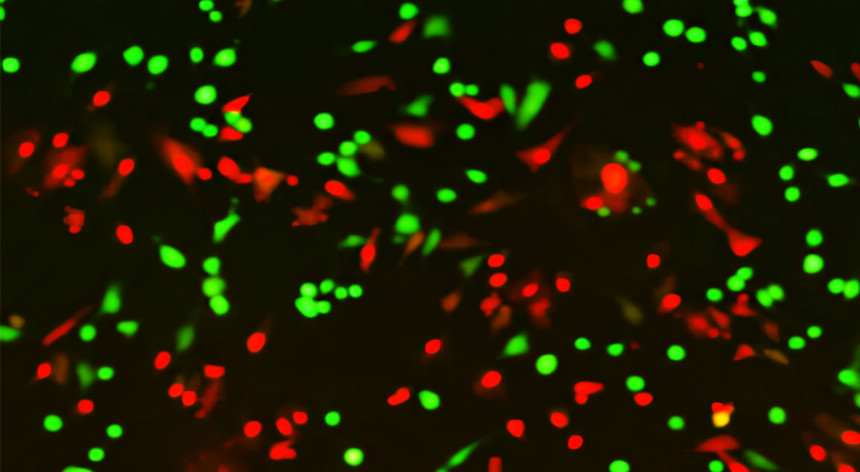
3.3 View the labeled cells under the fluorescence microscope.
Flow Cytometry Protocol: Viability Assay:
Troubleshooting1.1 Allow all reagents to come to room temperature.
1.2 Make an 80-fold dilution of Green Stock in DMSO working solution:(50 μg/mL, i.e., add 2 μL of Green Stock to 158 μL DMSO). The working solution should be used within one day.
1.3 Prepare a 1 mL suspension of cells with 0.1 to 5 ×106cells/mL for each assay. Cells may be in culture medium or buffer.
1.4 Add 2 μL of 50 μg/mL Green Stock DMSO working solution and 1 μL Red Stock solution to each mL of suspension cells solution . Mix the sample. Incubate the cells for 15-30 minutes at room temperature, protected from light.
1.5 As soon as possible after the incubation period (within 1-2 hours), analyze the stained cells by flow cytometry using 488 nm excitation and measuring green fluorescence emission for Green Stock (i.e., 530/30 bandpass) and red fluorescence emission for Red Stock (i.e., 610/20 bandpass). Gate on cells to exclude debris. Using single color-stained cells, perform standard compensation. The population should separate into two groups: live cells will show green fluorescence and dead cells will show red fluorescence.
- Dye deterioration:The live cell stain (Green Stock solution for live cell (Green label, brown vials)) will not deteriorate under normal storage conditions. If it is deteriorated, it will produce strong fluorescence, resulting in a decrease in sensitivity.
- For other questions, please contact our technical staff.